Acids 'n' Bases
·
Try some
practice worksheets
On this tutorial page you can find the following:
- Definitions of acids and bases
- Properties of acids and bases
- Discussion of the pH scale and pH calculations
- Titrations
- Miscellaneous other stuff
|
What are acids and bases? Definitions and Properties You hear about acids all the time. Acid reflux disease causes some people to have to take acid reducing medication. The fact of the matter is that you hear the word "acid" all the time. Most of us, however, don't have any idea what an acid is. The Bronsted-Lowry definition of acids are that acids are compounds that give off H+ ions when you stick them in water. This definition also says that bases are compounds that can accept H+ ions when you stick them in water. The Arrhenius definition of acids says
that they're compounds that give off H+ ions in water and that bases are
compounds that give off These definitions are the same.
Basically, if you've got something that can give off H+ in water, it's an acid.
As a result, all acids you'll be seeing in class have hydrogen atoms on them
that are ready to go jumping off in water. Most common acids have the
letter H in the beginning of the formula, with the exception of acetic acid
(it's at the end, for reasons we won't go into here). Bases, on the
other hand, are compounds that give off You can also define acids and bases as
being "strong" or "weak". Strong compounds are
compounds that completely break up in water. In other words, if we're
talking about a strong acid, all of the H+ ions break away from the molecule
in water. For strong bases, all of the There is a difference between a "strong" acid and a "reactive" one. Strong acids are all reactive, but some "weak" acids can also be extremely reactive. A good example of a weak, reactive acid is hydrofluoric acid, HF. I had a friend of mine who had a tube full of HF explode in his face - even though it's a weak acid, he still spent a long time recovering and suffered permanent scarring. Ask your teacher sometime which acid they'd rather put their hand into, HCl (a strong acid) or HF (a weak acid). If your teacher knows anything at all about acid chemistry, they'll reply HCl. Here are a couple of charts which show the
most common acids and bases. Some are strong and some are weak, as
indicated.
Properties of acids and bases Properties of acids include the following:
Properties of bases include:
|
|
The pH Scale Everybody has heard of pH. You've seen it in middle school, you've heard people talk about it in shampoo commercials, and you can even buy deodorant that's "pH balanced", whatever that means. Unfortunately, most people don't know what pH is. pH is a measurement of the H+ concentration in a liquid. If there's a high H+ concentration, the pH indicates that you've got a very acidic solution. If the solution is neutral, there's only a small H+ concentration, and the pH reflects that. If the solution is basic, there's almost no H+ concentration, and you can tell that by the pH number. pH is nothing more than a way of telling how concentrated an H+ solution is. Here's something people always have problems with: If I have a neutral or basic solution, how come I can measure the pH? After all, you can only measure pH if there's H+ ions present in a solution, and shouldn't the H+ concentration be 0 for neutral and basic solutions? Good question. It turns out that
water has the funny property that it tends to spontaneously break up into H+
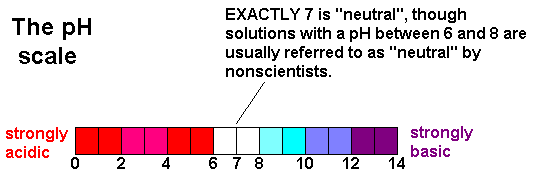
and Now that I've described what the pH scale is, let's take a look at it:
As you can
see, pH values between 0 and 7 are acidic and pH values between 7 and 14 are
basic. pH values of exactly seven are called
"neutral" solutions - if the pH is 6.99 it's an acidic solution and
if it's 7.01 it's basic. However, people usually refer to solutions
with a pH between 6 and 8 as being "neutral" because they're mostly
neutral. Don't put this on a test, though, because it will be marked
WRONG as it's technically wrong. pH Calculations OK. We're getting to the part with the calculations here. Now that we know what the pH scale is, let's learn how to compute the pH of a solution. The equation you need for these calculations is simple: pH = -log[H+] In this equation, [H+] refers to the molarity of the acid in the solution you're looking at. As a result, you'll need to be given (or calculate) the concentration of acid present before you can do this problem. Once you know this, it's just a matter of plugging this equation into your calculator. I'll show you how to do this in an example. Example: What's the pH of a 0.05 M solution of HCl? Solution: To solve
this, you only need to realize that in this case [H+] = 0.05. After
that, it's just a matter of plugging this thing into your calculator.
Let's learn how to do that.
If you don't have a scientific calculator, you're kind of out of luck, unless you have a log table (which nobody ever does, because if you need a log table you usually go buy the $10 calculator rather than the $30 log table, which is a lot less useful). As your math teacher if you can borrow a scientific calculator, borrow one from a friend, or keep bugging your parents until they give you ten bucks to buy a scientific calculator. For the record, don't tell your parents that I told you to get a graphing calculator - in my opinion, graphing calculators are useless for chemistry classes because you never actually need to graph anything in calculator format that you wouldn't also write down on a piece of paper. |
|
Titrations You've heard this word before. Come on, admit it. Your teacher talked about this in class and you didn't have any idea what he/she was talking about so your decided to pretend like it didn't exist. Well, I'm here to tell you it does. Might as well keep reading. Titrations are not all that hard to understand. In fact, the word "titration" comes from the Greek titros which means "to figure out the molarity of an acid or base solution" and the Latin ations which means "by neutralizing it with a solution whose concentration you already know". Those ancient people really had a way with words. Here's the idea. Let's say that you had really bad eyes and wanted to see how many toothpicks you had in a pile. In fact, your eyes are so bad that you can't even see the toothpicks to pick them up, much less count them accurately. This poses a problem. Your friend has an idea. You've got a bunch of little sandwiches lying around the house from the dinner party your parents hosted last night. If your friend sticks one toothpick into each sandwich, you could figure out how many toothpicks you had because all you'd need to do is count the number of sandwiches. You wouldn't be measuring the number of toothpicks directly by counting them, you'd be measuring them secondhand by how they interacted with something else. That's what a titration is. Let's say you have an acidic solution and wanted to figure out the molarity. Well, you can't do that directly, because you can't count acid molecules. They're too small. You can, however, make a basic solution with a concentration that you already know. If you keep adding base to the acid, eventually all of the acid molecules will be neutralized and the solution will turn from an acid to a base. If you know how many base molecules you added to the solution before the solution gets neutralized (and you will, because you'll add the solution drop-by-drop), you can figure out how much acid was in the solution in the first place. Of course, this leads to an interesting problem: How can you tell when the solution gets neutralized? The answer: Indicators! Indicators are chemical compounds that turn different colors when they're in solutions with different pH's. The indicators you'd most likely work with turn color when the solution becomes neutralized. Litmus, for example, is red in acid solutions and blue in basic solutions. Phenolphthalein (pronounced fee-no-thay-leen) is clear in acid solutions and pink in basic solutions. OK. Now that you have the basic idea behind titrations and know what indicators are, let's figure out how to solve some problems. The basic equation you need is this: M1V1 = M2V2
Example: If it takes 55 mL of 0.1 M NaOH solution to neutralize 450 mL of a HCl solution of unknown concentration, what's the molarity of the acid? Solution: Before
you can do anything, you need to translate this into something that makes
sense. Let's go through it slowly.
Now, all we need to do is plug it into the equation: (X)(450 mL) = (0.1 M)(55 mL)
And that's your answer! |
|
Miscellaneous other stuff In this section, I'm just going to talk about a bunch of other miscellaneous stuff that's a little more advanced. I'm not going to pretend that these things are especially complete or complex - they're mainly just here to fill in some of the main ideas I may have missed. If I don't talk about something in detail, it's either because I couldn't figure out a good way of explaining it over the computer, it's obscure enough that I don't think that all that many people would really need to know about it, or I just forgot about it when I was writing this section up. Whatever the reason, if it ain't here you should ask your teacher for help. Conjugate acids and
bases You may be asking yourself,
"What the heck does that mean?" Well, settle down As a general rule of thumb, the conjugate bases of strong acids are weak. For example, Cl- is the conjugate base of hydrochloric acid. If you wanted to, you could go swimming in a large body of water containing lots of Cl- and never get hurt. As a matter of fact, the ocean is full of Cl- (it comes from NaCl). So are you. Buffers Buffers are formed when you have a weak acid and its conjugate base present in the same place. If you wanted to make an acidic buffer, you'd place some acetic acid into a container with some sodium acetate. Voila! You have a buffer. If you want a basic buffer, just put a weak base into a container with it's conjugate acid. Same deal. Your blood is a buffered solution. If it wasn't, your pH would be go way down every time you had a soda and way up whenever you took some Tums. It's not a very handy survival technique to die every time you have a soda. |
As my favorite band says
at the end of an album, "That's all the singing." If you've got any questions or comments, email
them to me at misterguch@chemfiesta.com
and I'll do my best to get back to you.