Energy Diagrams
·
Try some
practice worksheets
In this Helpdesk article, you'll learn everything you ever wanted to know (and more!) about energy diagrams.
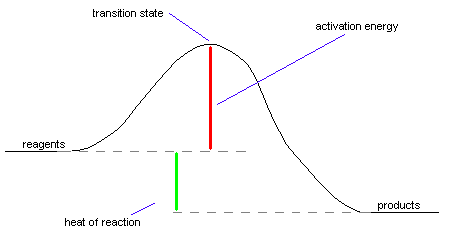
Here's what a sample energy diagram looks
like (this is for an exothermic reaction):
|
|
Important Terms reagents: What you start with in a reaction. |
Now, let's talk about what all that stuff
means!
|
What happens during a chemical reaction Chemical reactions require a bunch of stuff to take place before they can occur. Let's talk about all of the steps here, and what they mean in terms of the energy diagram above. What needs to be done to start a chemical reaction: Before a chemical reaction can take place, we need to do a few things. First of all, we need to assemble the ingredients that we want to make form our products. These ingredients are called reagents (sometimes referred to as "reactants"). Obviously, if we don't have ingredients, we won't be able to make anything! Sometimes just putting ingredients together is enough to start a reaction. However, in a lot of cases, we need to add a little bit of energy to get the reaction going. This isn't really surprising - after all, if you bake a cake, it's not enough to make the batter and just wait around for the reaction to occur. You need to stick the batter in the oven so enough energy goes into it to make it into a cake. Well, the same thing happens with many chemical reactions - if you want the reagents to make your desired products, you'll need to add some energy to get the process moving. This energy is called the activation energy, because the chemical process won't activate until the energy is added to it. Some reactions have higher activation energies than others, but all have an activation energy of one type or another. For example, when you put gasoline in contact with oxygen, you know that you shouldn't let any sparks near it. This is because the activation energy for the combustion of gasoline is very low, and even the smallest amount of energy will get it started. On the other hand, if you want to make calcium carbonate turn into calcium oxide and oxygen, you need to heat it to about a thousand degrees Celsius. Because a lot of energy is required to make this reaction start, we'd say it has a very high activation energy. Reactions that proceed quickly generally
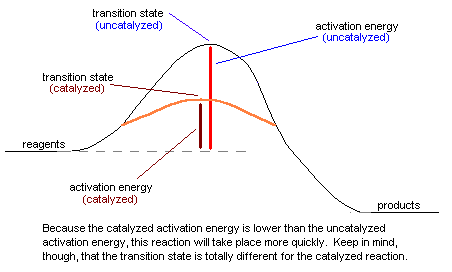
have lower activation energies than slow reactions. We're halfway there! The transition state: When we do a chemical reaction, the molecules that react come into contact with each other in such a way that they react. When the molecules are stuck in just the right way, they start to make the desired chemical change. The point at which the molecules have done exactly half of the change is referred to as the transition state. A good analogy to the transition state takes place whenever you go to the store to buy a candy bar. What happens is that you give the cashier the candy bar and they scan it on that little laser thingee. At some point during the transaction, you hand the cashier the money for the candy bar and he gives you the candy bar. That very instant in which you're giving the money to the cashier with one hand while grabbing the candy bar with the other is the transition state. At that instant, the cashier has halfway grabbed your money and you've halfway grabbed your candy bar. From that point on, the transaction practically completes itself. In chemical reactions, once the reagents have gotten past the transition state, they move smoothly to become products. Reactions with high activation energies occur more slowly than reactions with low activation energies. This happens because to make these reactions occur requires that the reagents have a lot of energy before they're able to reach the transition state. To go back to the candy bar analogy, imagine that you're trying to buy a candy bar from your worst enemy, and because he hates you so much, he doesn't want to sell it to you. If you're able to buy the candy bar at all, it'll be a slow process, because you'll have to expend a lot of energy trying to convince your enemy to sell you the candy bar in the first place. Eventually, if you spend enough time and energy, you'll be able to buy your candy bar - however, this will still take a long time. Now, imagine that you don't want to spend a lot of time getting your enemy to sell you a candy bar. Is there a way that you can get the candy bar more easily? Sure! Just get a friend your enemy doesn't hate to buy it for you. Because your friend can buy the candy bar without all of the hassle you have to go through, the transaction will take place more quickly. However, the transition state for this reaction will be different - instead of you handing the money to the cashier, your friend will be handing money to the cashier. This kind of situation is exactly the same thing that occurs when a catalyst is used to speed up a chemical reaction. For one reason or another, some chemical reactions simply don't take place quickly. As a result, it's handy to speed the reaction using a catalyst whenever possible. What the catalyst does is to help the reagents combine in such a way to become products, frequently by sticking them together in just the right way to react. Though the same products will be formed, the transition state is different because the catalyst is involved in the transaction. When the reaction is over, the catalyst is unchanged. It didn't react, it just helped along the chemical reaction. Take a look at this picture for a better idea of what a catalyst does:
The exciting conclusion: Formation of the products After you've gone past the transition state in a reaction, it pretty much goes right on down to products without too much trouble. After all, for the reaction to go backwards, you'd have to climb from the products back to the transition state, which would require a lot of energy again. Let's face it, would you want to return the candy bar to your enemy after having gone to all the trouble of convincing him to sell it to you? Of course not! If the products have lower energy than the reagents, the reaction is said to be exothermic. Exothermic reactions give off heat, causing the things that were formed to have less energy than they did before they reacted. It may seem weird to say that something that gives off heat would have less energy than something that doesn't, but let's consider yet another analogy. Let's say that for some reason or another, you've decided to go lie on a block of ice in your swimsuit. From the perspective of the block of ice, you're awfully warm. However, from your perspective, you're losing energy like crazy to the block of ice. When the ice has melted all the way, you'll have a lot less energy than you did before. That's how something that feels hot loses energy. If the products have greater energy than the reagents, the reaction is endothermic. Endothermic reactions absorb heat, making them feel cold. Again, let's say that you're lying on this block of ice. From your perspective, the block of ice feels very cold. Why is this? It's because the ice is absorbing all of your energy from you. The water actually has more energy than it did before you sat on it in ice form, because it absorbed all that energy from your body. Generally, endothermic reactions take place more slowly than exothermic reactions, because their activation energies are higher. Heat of reaction, or DH, is just a measure of how exothermic or endothermic a reaction is. A reaction that's very exothermic would have a very negative DH value, while a reaction that's only slightly exothermic would have a slightly negative DH value. Heats of reaction aren't affected at all by the addition of a catalyst to the reaction, because no matter how it happens, the result of the reaction is the same. After all, if your friend buys a candy bar for you, in the end, you still have the same candy bar as you would have had if you'd argued with your enemy to get it. |
Here's something for you to do to see if you're a pro: Sketch the energy diagram for an endothermic reaction, then compare it to the energy diagram that you can find by clicking HERE. If they're the same, then you know what you're doing!
- Questions? Comments? Email them to me at misterguch@chemfiesta.com
- Want to see something stupid and amusing? Click here.