How can I balance an equation?
![]()
·
Try some
practice worksheets
|
Why do we need to balance equations, anyway? Remember that at the beginning of the year, we did a lab where we added baking soda to vinegar and collected the whole mess in a rubber glove? Well, most of you were able to show that the mass of the stuff that we made was the same as the mass of the stuff we started with. (If you weren't one of those lucky people, then let me be the first to tell you this: The mass of the stuff that you make in a chemical reaction is the same as the mass of the stuff that you start with). This is called the Law of Conservation of Mass. Now, this shouldn't really be all that surprising, considering that this is true for most everything else in life. For example, when I make my world-famous chili, the weight of the chili that I make is the same as the weight of all the ingredients added together. As it is with chili, so it is with chemical reactions. Now, when we write chemical equations, we need to have the formulas for the reagents on the left side (the stuff that's going to do the chemical reaction) and the formulas for the products (the stuff you make) on the right. If we were to simply put the formulas of the chemicals on the left and right without saying how much of it was going to react, then we would run the risk of saying that the mass of what we end up with is different than the mass of what we started with. This would be the same thing as writing a recipe where we didn't specify how much of each ingredient is needed to make the chili. The bottom line: You need to balance the equations by sticking numbers in front of the chemicals on the left and right sides of the equation, like it or not. How can you do this? Check out the next section, titled... |
![]()
|
... Balancing Chemical Equations OK. You know why you need to balance chemical equations, but you don't yet know how to do it. It turns out that I'm star who knows how to explain things in a way that even the dumbest people know how to follow. And, hey, if the dumbest people can figure it out, so can you! Listen: There are four easy steps that you need to follow to make this work. Here they are: 1. Get yourself an unbalanced equation. I might give this to you, or I might make you figure it out. Either way, if you don't have an equation with all the chemical formulas and the arrow and all that other stuff, then you're out of luck. 2. Draw boxes around all the chemical formulas. Never, ever, change anything inside the boxes. Ever. Really. If you do, you're guaranteed to get the answer wrong. 3. Make an element inventory. How are you going to know if the equation is balanced if you don't actually make a list of how many of each atom you have? You won't. You have to make an inventory of how many atoms of each element you have, and then you have to keep it current throughout the whole problem. 4. Write numbers in front of each of the boxes until the inventory for each element is the same both before and after the reaction. Whenever you change a number, make sure to update the inventory - otherwise, you run the risk of balancing it incorrectly. When all the numbers in the inventory balance, then the equation can balance, and you can relax and enjoy a delicious bowl of Mr. Guch's chili. Read on for an example... |
![]()
|
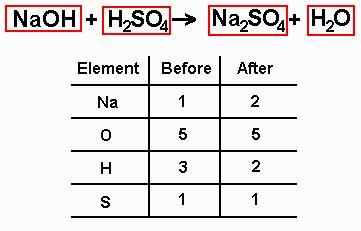
An example of equation balancing: Let's say I ask you the following thing on a test: "Balance the equation that takes place when sodium hydroxide reacts with sulfuric acid to form sodium sulfate and water." How do we solve this using the steps above? 1. Get yourself an unbalanced equation. Here's where you use your knowledge of formulas to help you out. If you know what the formula of sodium hydroxide, sulfuric acid, sodium sulfate, and water are, you'd be able to write the following unbalanced equation:
2. Draw boxes around all the chemical formulas. This is the step that people frequently don't do because they feel that it's a stupid thing to do. Those people are morons. Ignore them. You're drawing those boxes so that you'll be sure not to mess around with the formulas to balance the equation. While they all suffer in the pits of academic hell, you'll be laughing from the honor roll. Here's what the equation looks like:
3. Make an element inventory. In this inventory, your job is to figure out how many atoms of each element you have on the left and right sides of the equation. Now, if you look at the equation, you should be able to see that on the left side of the equation there is one sodium atom, five oxygen atoms (one from the sodium hydroxide, four from the sulfuric acid), three hydrogen atoms (one from the sodium hydroxide, two from the sulfuric acid), and one sulfur atom. On the right side of the equation, there are two atoms of sodium, one atom of sulfur, five atoms of oxygen (four from the sodium sulfate and one from the water), and two atoms of hydrogen. Thus, your element inventory should look like this:
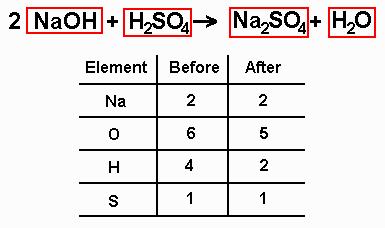
4. Write numbers in front of each of the boxes until the inventory for each element is the same both before and after the reaction. Now, what happens when we put a number in front of a formula? Basically, anything in that box is multiplied by that number, because we're saying that we have that many of that kind of molecule. So, looking at the inventory, what should we do? Well, we can see that on the left side of the inventory, there is one atom of sodium and on the right there are two. The solution: Stick a "2" in front of the sodium hydroxide on the left side of the equation so that the numbers of sodium atoms are the same on both sides of the equation. When we do this, the new atom inventory should look like this: (I'll let you figure out how this is done)
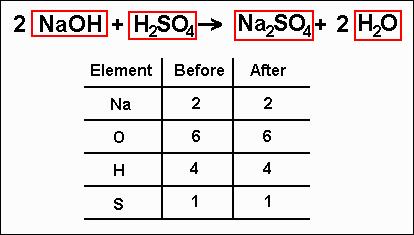
Now what? Well, looking at the new inventory, we can see that we now have two sodium atoms on both the left and the right sides, but the others still don't match up. What to do? You can see from the inventory that on the right side of the equation, there are two hydrogen atoms and on the left there are four. Using your amazing powers of mathematics (and hopefully not needing to use a calculator), you can see that two multiplied by the number two becomes four. That's what you need to do. How? Put a "2" in front of the water on the right side of the equation to make the hydrogens balance out. Now that this is done, you should make a new inventory that looks something like this:
Since both sides of the inventory match, the equation is now balanced! All other equations will balance in exactly the same way, though it might take a few more steps in some cases. |
![]()
|
Problems you might encounter: Is it all as easy as I made it look above? Well, yes and no. Yes, it should work all the time. No, sometimes you need to do some tricks to find the right numbers to add into the equation. For example, what happens when you do the inventory, and you find that there are two atoms of element X on the left side of the equation and three on the right. How can you make those match? When you run into this problem, find the lowest common denominator of those two numbers, and then put the numbers in front of those two boxes which allow the inventory on both sides to match. In the element X example, the lowest common denominator of two and three is six, so you'd put a "3" in front of the molecule on the left, and a "2" in front of the one on the right. Element X will then match up, and you can use a new inventory to see what else needs to be done. Another common problem: What happens when the only way you can get a problem to work out is to make one of the numbers a decimal or fraction? When this happens, find the largest molecule in the equation and stick a "2" in front of it. Then start the problem over. Will this work all the time? Well, no. But it will work sometimes, and give you a new strategy for hard problems. Most importantly: Always remember to keep the inventory of the elements current! If you try to keep it in your head, you'll screw it up. Nobody can keep a bunch of changing numbers in their head for very long. I certainly can't, and you can't either. |
![]()
|
Some practice problems: Here are some practice problems. The solutions are in the section below this one. 1. __NaCl + __BeF2 --> __NaF + __BeCl2 2. __FeCl3 + __Be3(PO4)2 --> __BeCl2 + __FePO4 3. __AgNO3 + __LiOH --> __AgOH + __LiNO3 4. __CH4 + __O2 --> __CO2 + __H2O 5. __Mg + __Mn2O3 --> __MgO + __Mn |
![]()
|
Solutions for the practice problems: 1. 2 NaCl + 1 BeF2 --> 2 NaF + 1 BeCl2 2. 2 FeCl3 + 1 Be3(PO4)2 --> 3 BeCl2 + 2 FePO4 3. 1 AgNO3 + 1 LiOH --> 1 AgOH + 1 LiNO3 4. 1 CH4+ 2 O2 --> 1 CO2 + 2 H2O 5. 3 Mg + 1 Mn2O3 --> 3 MgO + 2 Mn |