All About Mole Calculations
·
Try some
practice worksheets
How to do calculations between moles,
atoms or molecules, and
grams of a substance
One of the main problems that beginning chemistry students have is in doing conversions between grams, moles, and molecules (or atoms). Usually, a question will be asked of you in the following form:
How many moles are in 22 grams of copper metal?
If you're confused by this problem, don't worry. Most people are when they start doing this kind of problem. To make life easier for you, I put together a "road map" which tells you exactly what you need to do to convert between atoms (or molecules), grams, and moles.
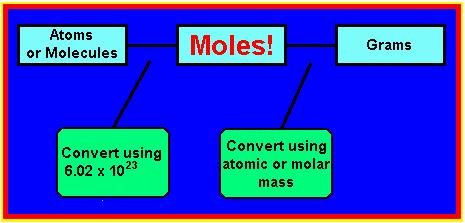
You should read this picture the same as you
would read a subway map. For example, if you want to go from
Example: How many moles are in 22 grams of copper metal?
In all problems like this, you need to go through four steps to find a solution.
The Four Steps to Solving Mole Problems:
Step 1: Figure out how many parts your calculation will have by using the diagram
Looking at the diagram above, we can see that we are going between grams and moles, which is a one-step conversion. Furthermore, we can see that we need to use the atomic mass of copper as our conversion factor.
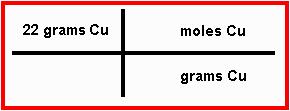
Step 2: Make a T-chart, and put whatever information the problem gave you in the top left. After that, put the units of whatever you were given in the bottom right of the T, and the units of what you want to find in the top right.
In this case, the problem gave you "22 grams of copper" as the starting information. Because this is what you were given, put "22 grams of copper" in the top left of the T. Since "grams of copper" is the unit of what you were given, put this in the bottom right of the T. Since you want to find out how many moles of copper are going to be made, put "moles of copper" as your unit in the top right. When you've done this, your calculation should look like this:
Step 3: Put the conversion factors into the T-chart in front of the units on the right.
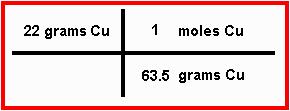
As we saw from the "map", the conversion factor between grams and moles is the atomic mass of copper. Because we measure atomic mass in grams, you need to put the atomic mass in front of the unit "grams of copper". What do you put in front of moles? Whenever you do a calculation of this kind, you need to put "1" in front of moles, like you see here:
Step 4: Cancel out the units from the top left and bottom right, then find the answer by multiplying all the stuff on the top together and dividing it by the stuff on the bottom.
In this case, you'd multiply 22 by one and divide the result by 63.5. Your answer, 0.35 moles of copper:

And that's how you do a one-step problem of this kind!
Solving Two-Step Mole Calculation Problems:
What happens if we need to solve a problem that requires we not just go from one box in the next in our diagram, but across the entire diagram? Well, it means that we need to do two steps in our calculation. Let's see that "map" again to see what I mean:

If we were asked to convert 22 grams of copper to atoms of copper, we'd have to go from one end of the map to the other. Instead of doing a simple one step calculation, we'd need to do a two-step calculation, with the first step going from grams to moles and the second step going from moles to atoms.
How can we solve this kind of problem? Well, we start off by doing the same thing that we did in our last example: We had to convert grams to moles before, and we can see from the map that we have to convert grams to moles now, too. To refresh your memory, here's the calculation from last time:

In the next step, we do the same thing over again, except that we need to add another T to the T-chart. When you do this, take the units of the thing at the new top left and put them on the bottom right (in this case, moles). Then take the units of what you want (in this case, atoms) and put it in the top right. Finally, put in your conversion factors, which from the chart above is Avogadro's number, or 6.02E23. Since this number refers to the number of atoms in a mole of a substance, we put this in front of "atoms of copper". Again, put the number "1" in front of moles, because we're saying that there are 6.02E23 atoms in ONE mole of an element.
When we add all these terms in, we can cross out the units that cancel out, as shown. To get the answer, multiply all the numbers on the top together and divide by the numbers on the bottom. Your answer should then be set up like this:

And that's how you do mole problems!
Comments, questions, or gripes? Email me at misterguch@chemfiesta.com